


This label reading information is in line with the new Plain English Allergen Labelling (PEAL) legislation. As of February 2024, most products now comply, however longer shelf-life products have until February 2026 to transition. PEAL requires specific terminology in the ingredient list and mandates allergen summary statements.
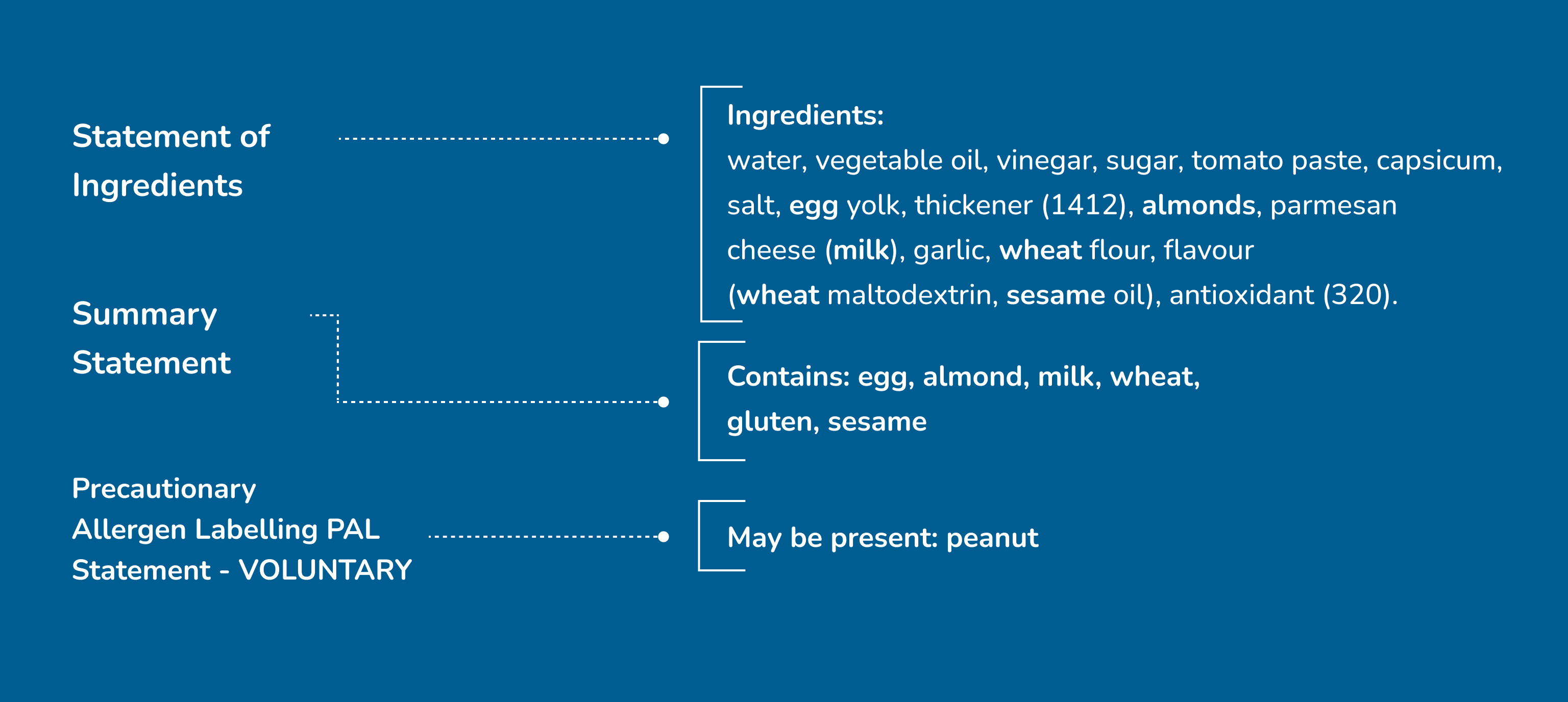
Statement of Ingredients – MANDATORY
Every time an ingredient is derived from wheat, rye, barley or oats, this will be declared, in bold, in the statement of ingredients. If there is no mention of wheat, rye, barley or oats in the ingredient list, this means that no ingredient is derived from a gluten-containing grain.
Summary Statement (‘Contains…’) – MANDATORY
Mandatory summary statements provide a summary of the allergens (including gluten) present in the ingredients of a product. Summary statements help us to identify which allergens are present in a product; the source ingredient of any allergen will also always be listed in the statement of ingredients. Summary statements will appear as Contains: ..” The food code requires that the terms ‘gluten’ and ‘wheat’ be used, and that they be declared separately in the allergen summary statement. This is to make labelling clear and helpful for both those with coeliac disease and those with wheat allergy.
‘Contains: Wheat, Gluten’
Gluten and wheat must be declared separately in the allergen summary statement. The inclusion of gluten in the allergen summary statement indicates that gluten is present. These products are not suitable for the gluten free diet.
‘Contains: Gluten’
If an ingredient in the product contains gluten, ‘gluten’ will be listed in the Allergen Summary Statement: ‘Contains: Gluten’. These products are not suitable for the gluten free diet.
‘Contains: Wheat’
This indicates that the product contains a wheat derived ingredient, but that the wheat derived ingredient does NOT contain gluten. If you see a declaration of ‘Contains: Wheat’ (in the absence of ‘Contains: Gluten’), the product is suitable. This declaration may appear on products that are labelled ‘gluten-free’.
Exceptions
Some ingredients that are wheat derived are highly processed, rendering them suitable for the gluten free diet. The following ingredients are suitable for the gluten free diet, even though they are wheat derived.
Common wheat derived ingredients that are suitable:
-
Wheat derived glucose
-
Caramel Colour (150) from wheat
-
Dextrose from wheat
Other wheat derived ingredients that are suitable:
-
Fructose (wheat)
-
Maltose (wheat)
-
Sorbitol (wheat)
-
Maltitol 965 (wheat)
-
Gluconodelta-lactone 575 (wheat)
-
Glutamate based flavours 620-625 (wheat)
Special note about glucose:
Wheat derived glucose is exempt from mandatory allergen declaration under specified circumstances. This means that food manufacturers are not required to state on packaging that glucose syrup is wheat derived if detectable gluten levels in the glucose syrup are below 20ppm. Coeliac Australia considers wheat derived glucose to be suitable for the gluten free diet.
What about numbered ingredients (food additives)?
Listed as numbers between 100 and 1521 in an ingredient list, manufacturers use a large range of additives to improve their product’s appearance, texture, composition, taste and shelf life. While some of these additives can be sourced from wheat or barley, most remain suitable (see note on ‘exceptions’). The only additives that should be avoided if derived from barley or wheat are:
234 (Barley) 1400 – 1450 (Wheat)
These numbers without barley or wheat after them have been derived from another (gluten free) source and are suitable.
Precautionary Allergen Labelling (PAL)… ‘Cross contact statements’ – VOLUNTARY
Some products use advisory statements, such as May be present: Gluten’,‘May contain wheat’ or ‘Manufactured on the same line as gluten containing products’ to indicate a risk of inadvertent contamination from gluten-containing products. PAL is often used unnecessarily. While the ‘safest’ approach is to avoid foods that use PAL, each individual should use their discretion when deciding whether to use such products.





